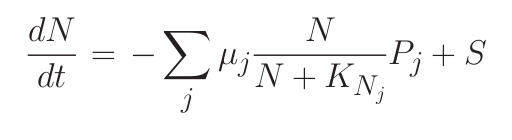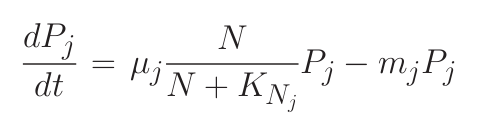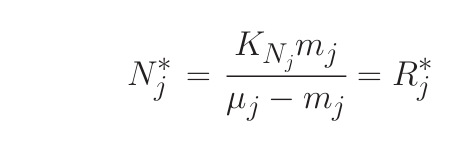Ecological Control of Subtropical Nutrient Concentrations
story by Helen Hill and Stephanie Dutkiewicz.
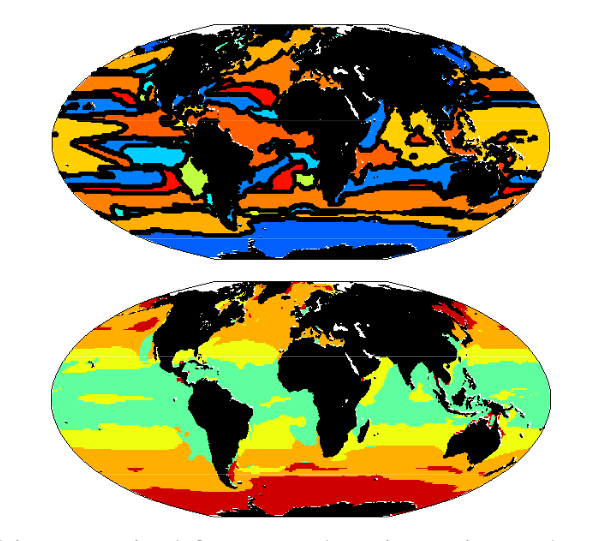
Figure 1: Multiple-Resource Experiment. (top) Emergent biogeographical provinces, defined by most dominant species, reminiscent of Longhurst (1995). (bottom) Biogeography of four major functional groups: (i) Diatom-analogs (red), (ii) other large phytoplankton (orange), (iii)Prochlorococcus-analogs (green), and (iv) other small phytoplankton (yellow-green).
In this article we spotlight recent work by Darwin Project team members Stephanie Dutkiewicz, Mick Follows and Jason Bragg, who have been examining the utility of resource control theory to interpret the relationships between organisms and resources in a global coupled physical-biogeochemistry-ecosystem model built around MITgcm.
The team find that in regions of low seasonality, resource competition theory (Tilman, ’77) not only anticipates the competitive outcome amongst organisms but also provides a quantitative diagnostic of ecological control of nutrient concentrations. DFB’s sensitivity experiments clearly indicate control on the ambient nutrient by phytoplankton physiology as predicted by the theory.
Ecological Framework
Consider a simple system where many phytoplankton (Pj ) are nourished by nutrient (N ) resupplied at rate S. Phytoplankton are lost at rate m, and have physiology controlled by growth rate (μj ) and nutrient half-saturation (KNj). The time rate of change of nutrient concentration and phytoplankton can be simply related through equations 1 and 2.
1) In steady state (“resource competition theory”):
where R* is the ambient concentration of limiting nutrient with which a population of phytoplankton would come to equilibrium.
According to this model:
- Nutrients are drawn down to a concentration in equilibrium with organisms which have the lowest R* thus preventing organisms with higher R* from being able to compete (K-selection); (Coexistence can occur if phytoplankton have the same R* in a perfectly steady environment).
- Nutrient concentration is determined by phytoplankton physiology and the tight coupling between growth and loss
- Phytoplankton concentrations are controlled by the balace between their losses and resource re-supply.
2) At the beginning of spring, in a highly seasonal region:
Growth rate matters most (r-selection). The organism with the fastest per capita growth under ideal resource conditions will exclude all others over several seasonal cycles.
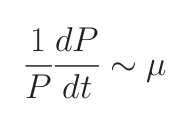 |
(5) |
Numerical Simulations
The team initialized the model simulations with 78 phytoplankton types (exhibiting an assortment of growth parameters) and 2 grazers (Follows et al, 2007, with minor modifications). This biogeochemical-ecosystem was then embedded in circulation state estimates, provided by the ECCO-GODAE consortium (Wunsch and Heimbach, 2007).
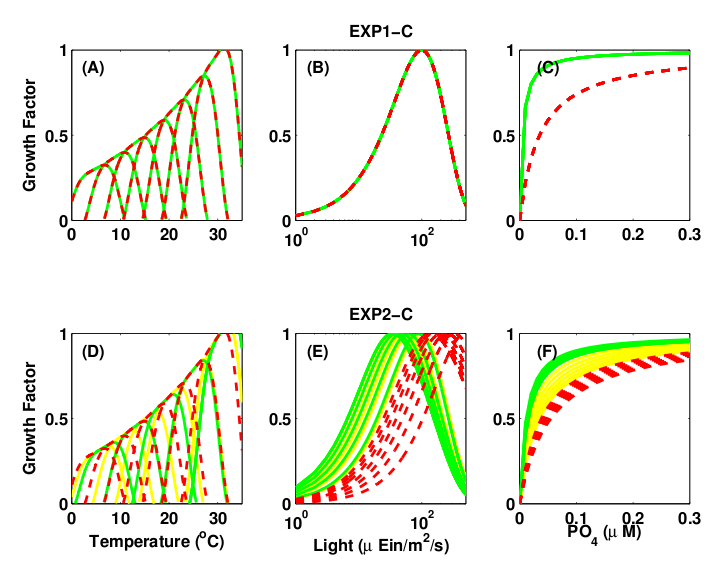
Figure 2: Growth μ = μmaxγT γI γN is function of temperature (γT), light (γI ), and nutrients (γN). (top) Single Resource Case, (bottom) Multiple Resource Case. Green and yellow: K-strategy phytoplankton types with low half-saturation constants and low μmax. Red: r-strategy phytoplankton types with high μmax and high half-saturation constants. Green: small phytoplankton that cannot use nitrate (Prochlorococcus analogs).
The team considered 2 experiments:
-
-
Single Resource Case:
-
One nutrient with phytoplankton divided into two groups one group having low R* (low KN, high μ) and one having a higher R* ( high μ, high KN). (K-strategy)
-
-
Multiple Resource Case:
-
N, P, Si and Fe cycling (ie multiple resources) with phytoplankton growth traits randomly assigned from within reasonable ranges (Fig. 2).
Single Resource Case
A look at behaviour in the single resource case helps illustrate the mechanisms at work in the more complex multi-resource case to follow:
What type lives where?
- r-strategy types dominate in high latitudes where there is high seasonality and high nutrients
- K-strategy types dominate in the low and mid latitudes.
Ecological Nutrient Control
|
Biogeography
|
Multiple Resource Case
How well does the theory hold for a more realistic simulation? The team
consider an experiment with with the cycling of N, P, Si and Fe and more strategy types of phytoplankton (e.g. additional nutrient limitation, differing light requirement, differing grazing potential)
Which types live where?
- Transition between regimes is gentler than in single resource case.
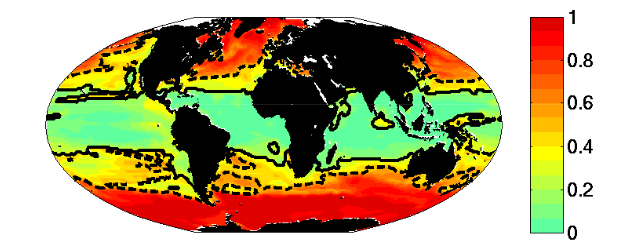
Figure 6: Multiple Resource case. Fraction of biomass in “large” (faster growing) relative to total biomass (compare to Fig. 3). Dashed line indicates the 0.5 contour. Solid line indicates the region where the Prochlorococcus-analogs dominate.
Ecological Nutrient Control
|
Biogeography
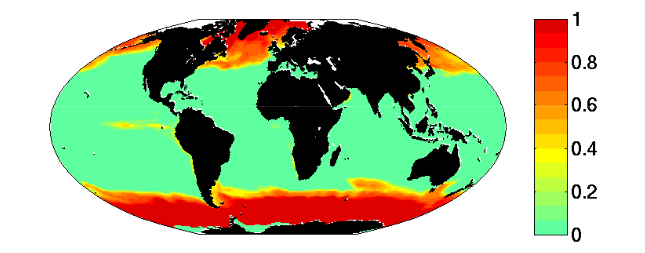
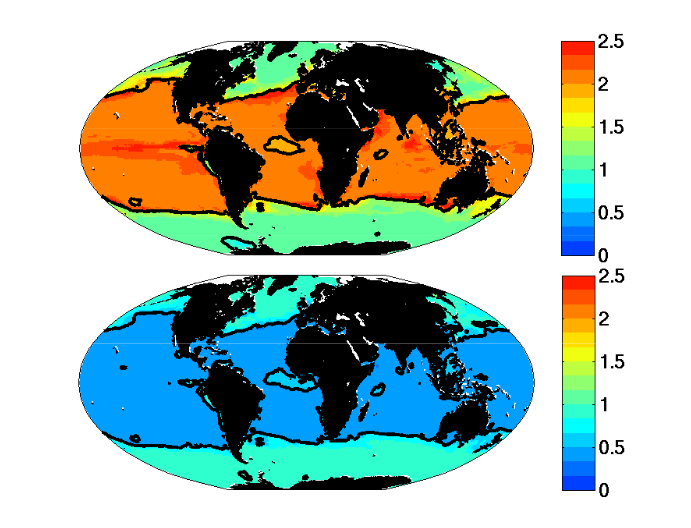
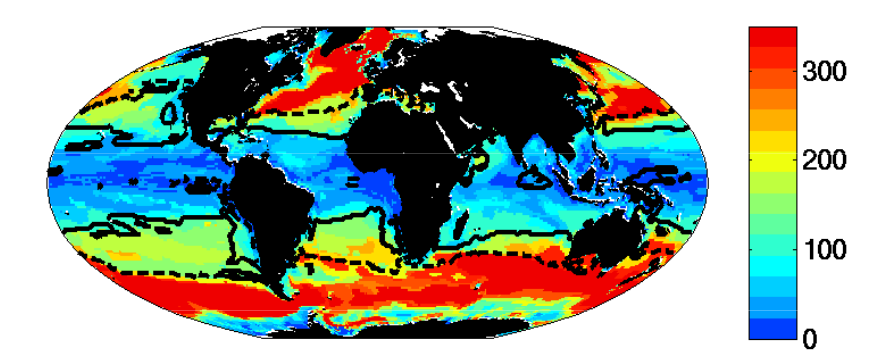
- The region where limiting nutrient appears controlled by the phytoplankton physiology is quantitatively similar to the Single Resource Case (annual range of mixed layer depth < 250m, Fig 5)
- Prochlorococcus-analogs have the lowest R*, but are also usable to utilize nitrate. These dominate (Fig 1) in the most stable sub-region (annual mixed depth range < 100m, Fig. 5). Annual temperature and PAR (photosynthetically active radiation) ranges are also small here.
In summary the team found that:
- The r and K strategy types dominate in the regions we would expect: where nutrient requirements (K) are low in the low/mid latitude regions of relatively low seasonal variability, and high growth rate (r) in the highly seasonal regions where nutrients are also high.
- In regions of relative stale physical environment, phytoplankton physiology has an important role in setting the biogeochemical environment.
- Links to ecological theory helped better understand the numerical solutions… and potentially the real marine environment.
Want to find out more? Contact Steph…
References
Longhurst, A. Seasonal cycles of pelagic production and consumption, 1995: Progress in Oceanography,36, 77, doi:![]() 10.1016/0079-6611(95)00015-1
10.1016/0079-6611(95)00015-1
Tilman, D. Resource competition between planktonic algae: an experimental and theoretical approach, 1977, Ecology 58:338-348.
Wunsch and Heimbach, Practical global oceanic state estimation, 2006: Physica D: Nonlinear Phenomena, 230, 197-208, doi:10.1016/j.physd.2006.09.040
Publications
Dutkiewicz, S., M.J. Follows and J. Bragg. Modeling the coupling of ocean ecology and biogeochemistry, 2009: Global Biogeochemical Cycles, 23, GB4017, doi:10.1029/2008GB003405. PDF.
Follows, M.J., S. Dutkiewicz, S. Grant and S.W. Chisholm, 2007: Emergent biogeography of microbial communities in a model ocean. Science, 315, 1843-1846, doi: 10.1126/science.1138544. Read.